Occupational areas
There are a number of possible positions at ZytoService, all linked by a quality requirement that sets standards.

Pharmaceutical Consultation
Pharmaceutical Consultation
It goes without saying for us that every incoming order is pharmaceutically tested before production. This testing is carried out by our team of pharmaceutical consultants. Our strict quality and safety standards are guaranteed through well-founded pharmaceutical advice and close coordination with the pharmacy placing the order, ensuring we achieve the optimum result for the patient.

Cleanroom Laboratory
Cleanroom Laboratory
Our cleanroom laboratories are at the heart of our company. Infusion solutions for the critically ill are produced on modern workbenches under sterile conditions. Whether at the workbench itself or in adjacent areas of work, such as laboratory cleaning or production preparation, each individual is trained by us in accordance with the strict GMP guidelines. In this way, we can ensure compliance with the highest possible quality standards for patient care.
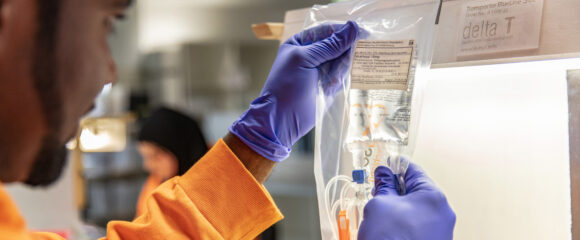
Quality Control
Quality Control
Strict quality control is a prerequisite for producing perfect infusion solutions. This takes place as early as in the incoming goods department by checking, testing and punctually providing the necessary raw materials.
Before delivery, the finished infusion solutions are tested thoroughly for integrity, particle content and purity in the final inspection area. Our strict, GMP-compliant control mechanisms create trust and transparency in the smooth production process.

Production Preparation & Logistics
Produktionsvorbereitung & Logistik
The picking team ensures the smooth supply of necessary active ingredients and auxiliary materials to the laboratories. Supply is carried out both by the team outside the clean room and by our laboratory logisticians, who ensure efficient material supply for the manufacturing process within the laboratory. The production control team coordinates optimal utilisation of the laboratories, taking pharmaceutical and economic aspects into consideration.
Finally, the dispatch team takes over, delivering the finished products to our pharmacies. Customers ensure proper interim storage and perfect transport conditions for our infusion solutions, coordinate the numerous shipments and control our logistics centre.

Quality Assurance
Quality Assurance
The manufacturing of our infusion solutions is governed by strict GMP quality and safety requirements (GMP = Good Manufacturing Practice). Our Quality Assurance department is responsible for designing, implementing and monitoring the entire quality management system.
Alongside the specialist departments, Quality Assurance is responsible for designing the quality management system. In addition, it designs and monitors training courses relevant to QM, risk management and microbiological monitoring of the entire manufacturing process. Every employee and the entire production area are checked daily for microbiological contamination. The samples are evaluated both in our own ZytoService control laboratory and in certified external GMP laboratories.




